01-11-2021 By Quick Biology
Cancer immunotherapy like chimeric antigen receptor (CAR) T cells has been approved and used in blood and bone marrow cancer. Although CAR-T therapy is much more powerful and promising in these types of cancers, long-term remissions are still observed in < 50% of patients.
In Nature Medicine, Deng et al. used multi-dimensional analyses to investigate the cellular signature of CAR T cell products in patients, they aimed to obtain information of such immunotherapy response and its toxicity (Fig. 1, ref1). Twenty-four patients with large B cell lymphomas (LBCL) are involved in their study. Deng et.al collected three layers of data.
[1] Gene expression signature: They performed whole-transcriptome sing cell RNA of 137,326 residual cells obtained from washing the standard-0f-care axi-cell product bags after infusion of the CAR T cells into patients. Single-cell TCR repertoire sequencing is also carried out.
[2] Clinical phenotype: Three-month PET/CT and toxicity grading of patients were investigated.
[3] cell-free tumor DNA profiling: Plasma was collected periodically to track the amount of cell-free tumor DNAs in patients.
By incorporated these data (Fig.2, ref2), they identified transcriptomic features associated with efficacy and toxicity in 24 patients with LBCL. Furthermore, a rare cell population with monocyte-like transcriptional features was associated (P = 0.0002) with high-grade ICANS (immune effector cell-associated neurotoxicity syndrome). Their data suggest that heterogeneity in the cellular and molecular features of CAR T cell infusion products contributes to variation in efficacy and toxicity after axi-cel therapy in LBCL, and that day 7 molecular response might serve as an early predictor of CAR T cell efficacy.
Figure 1: Understanding the diversity of responses to CD19 CAR T cells (ref1). The study was able to identify, through a multidimensional approach, that a response to CD19 CAR T cell therapy correlated with a specific signature at day 7, and that response and toxicity were linked to specific populations of cells found in the infusion product4. Parker et al. showed that neurotoxicity may also be mediated by a specific population of CD19+ mural cells that may be targets of CD19-directed CAR T cells5. IACs, ICANS (immune effector cell–associated neurotoxicity syndrome)-associated cells.
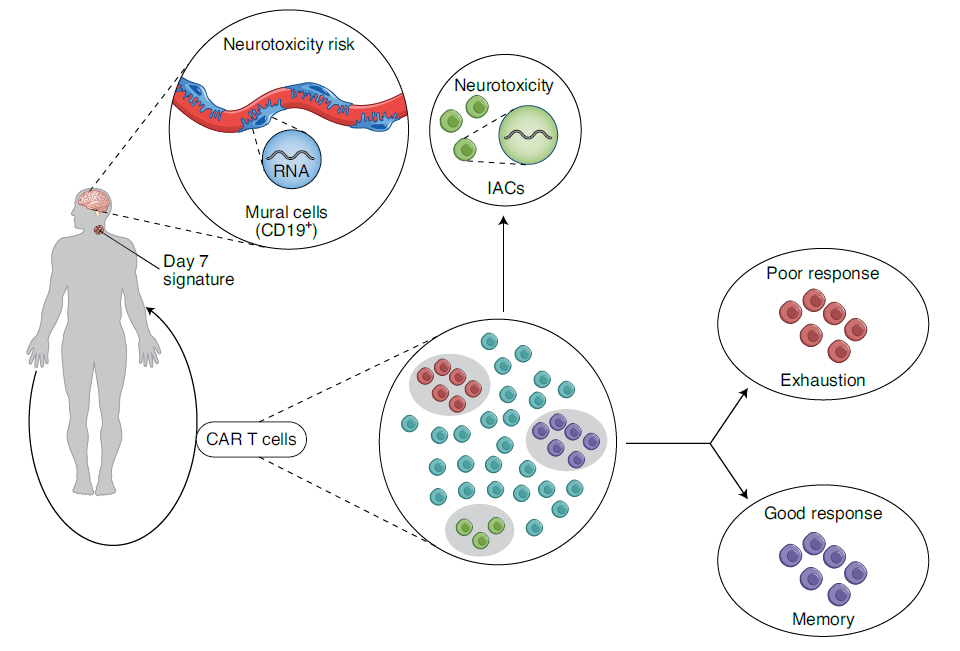
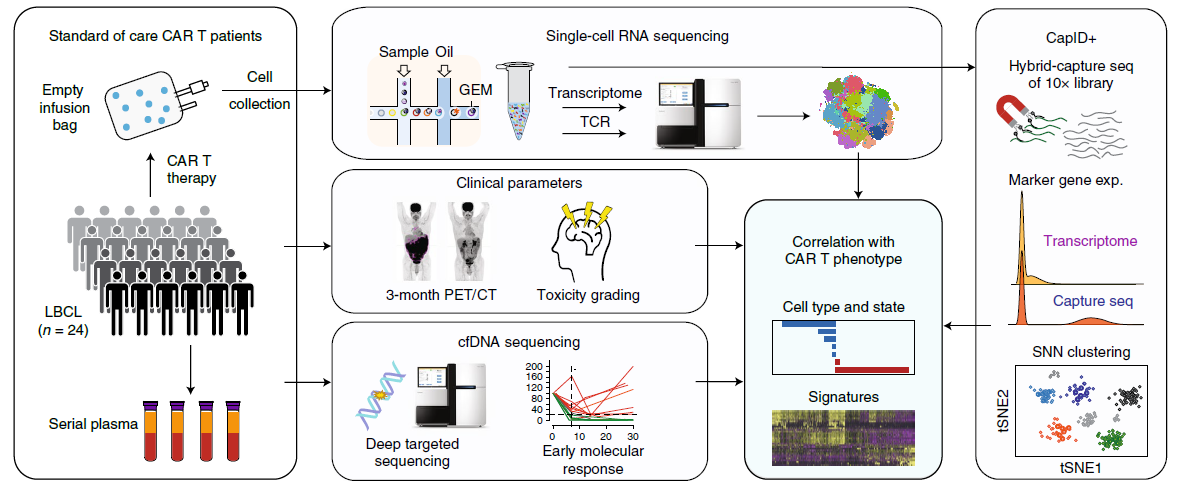
Figure 2: Overview of Deng’s study (Ref2). A schematic overview of the experimental design and bioinformatics flow for scRNA-seq analysis of 137,276 residual cells from CAR T cell infusion products of 24 patients with LBCL. The approach incorporated single-cell transcriptome profiling of CAR T cell infusion products boosted by CapID+, correlation of single-cell functional states and gene expression signatures with efficacy assessed by PET/CT and cfDNA sequencing and with toxicity assessed by clinical grading.
Quick Biology can assistant you with single-cell transcriptome or BCR/TCR sequencing. Find More at Quick Biology.
See resource:
- 1. Ramakrishna, S. & Shah, N. N. Using single-cell analysis to predict CAR T cell outcomes. Nat. Med. 26, 1813–1814 (2020).
- 2. Deng, Q. et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat. Med. 26, (2020).